For the estimated 3 to 4 million Americans with the Hepatitis C virus, a new class of drugs called Direct-Acting Antiviral Agents (DAA) appear to be a game-changer, offering better cure rates without the debilitating side effects of the traditional treatment of interferon injections. While these “miracle drugs” sound promising, they come with exorbitant price tags and their potential for long-term side effects remains unclear. Nonetheless, doctors and patients are excited that a true cure for Hepatitis C may finally be available.
“There are several new drugs that are really revolutionizing our ability to treat patients,” says Michael Saag, MD, professor of medicine at the University of Alabama at Birmingham, and Director of the Center for AIDS Research there. “Over the next 3 years we will see most every person who has HCV as a candidate for therapy, and when they start the medication, they have a 95% chance of being cured.”
Browse This Article
Targeted Treatment Yields Better Results
A total cure is what Joyce Firenza of Memphis, Tennessee, was hoping to achieve. She lived with Hepatitis C for 25 years and was well-acquainted with interferon’s side effects. In May 2014, she began a 3-month regimen of Sovaldi and Olysio, 2 of the new DAAs. Unlike interferon, which was injected, Sovaldi and Olysio are oral medications taken 1 each day for 12 weeks.
“This was unbelievable,” says Firenza who was told in August that she was disease-free. Three months later, the virus had not returned. “It’s hard to believe that this medication is strong enough to kill this virus, yet you don’t feel like you’re taking anything. That’s incredible to me.” She had no immediate side effects at all.
The 2 drugs that Firenza took, also known as simeprivir and sofosbuvir, are leading the way toward a new method for treating Hepatitis C, also known as HCV. Another medication, Harvoni, also known as a combination of ledipasvir and sofosbuvir, was approved in October 2014, and several others are in the FDA’s pipeline and expected to win approval.
What’s different about these drugs?
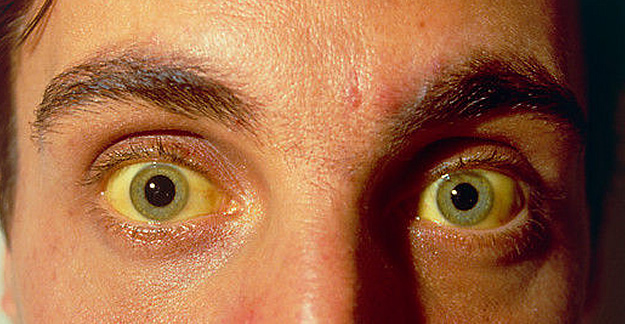
Until now, “the regimens to treat Hepatitis C were fairly toxic. Interferon was a revolutionary drug in its time and it was the best treatment we had. We used it for almost 2 decades, but only a very select group could tolerate it,” says Michael Fried, MD, professor of medicine and Director, UNC Liver Center at the University of North Carolina at Chapel Hill. The long list of potential side effects of the drug ranged from yellowing skin and eyes to diarrhea and and nausea, tooth and gum problems to hair loss, and on to more serious reactions like chest pain, seizures and depression. The medication was designed to replicate the interferon naturally found in the body, a protein that aided cell growth and boosted the immune system. On top of its pernicious side effects, interferon worked less than half of the time as a treatment for Hep C.
While interferon sought to improve the body’s immune response, these drugs are attacking the actual virus, Dr. Saag says. “The science has taught us what we need to know about the virus’s life cycle — how it enters a cell, how it replicates, and how the different life stages of the virus are targeted by these drugs,” he says. “When you attack the virus during its life cycle at 2 different locations, the virus stops reproducing. Cells that were infected with the virus die, and when the infected cells die, the virus dies with them. Over the 6- to 8-week period that this occurs, no new viruses are produced, and the liver can get on with the process of repairing itself. Then you’ve got a cured infection.”
A 60-year-old musician named John from Los Angeles, is also hoping the new class of drugs will provide a complete cure. He has had Hepatitis C for 40 years. Like Firenza, he’s had 2 courses of interferon treatment, and neither made a dent in his illness. He is about to start Harvoni, a combination pill that means that John will take only 1 pill a day for the duration of his treatment.
“This will dramatically change my life — take away the possibility that I will have cirrhosis, an enlarged spleen, liver cancer. I feel very good about it,” John notes.
Dr. Saag says he should. “It’s absolutely reasonable to expect that someone who takes this drug can stem the course of his disease. In addition, the chronic inflammation in the liver produced in response to the virus replicating is associated with higher rates of heart attacks, diabetes, kidney disease, and neurologic disorders,” he adds, noting that those risks are also reduced when the liver is repaired with these drugs.
Fewer Side Effects
Like most medications, DAAs do have side effects, which include fatigue, headache, and a mild skin rash. But Dr. Saag and Dr. Fried say that few of the patients they’ve treated with them have reported any effects at all.
‘The toxicities that occur in the 1 in a thousand category might not be seen until you treat 100,000 patients. But so far, these are highly effective and incredibly safe, but that is so far.’ — Dr. Henry Masur, NIH
“In the first analysis, these drugs are very effective and very easy to take,” says Henry Masur, MD, Chief, Critical Care Medicine Department at the National Institutes of Health Clinical Center. “Of course one always needs to be cautious. What you learn in the first 10 patients is a microcosm of what you know after you treat 100 patients,” Dr. Masur notes. “And the toxicities that occur in the 1 in a thousand category might not be seen until you treat 100,000 patients. But so far, these are highly effective and incredibly safe, but that is so far.”
Most everyone who is infected with HCV is a candidate for one of these new drugs, Dr. Masur says, adding that as more drugs come on the market, doctors and patients will have more options for prescribing and taking them. In the next year at least 3 more Hepatitis C drugs are expected to hit the market. “Each of these drugs is a little bit different,” he says. “Each has to be studied, but it looks like the interactions with other drugs are very few. We think for all patients with Hep C, there will be an option. It may not be the same option, but there will be an option.”
The biggest problem with DAAs at the moment is their cost. Harvoni, for example, costs $1,200 per pill. A course of Olysio and Sovaldi can run upwards of $160,000. “Insurance companies, Medicare and Medicaid are pushing back on providing the prescription owing to the cost,” says Dr. Saag. “Each entity has to negotiate a cost level with each pharmaceutical company. For those patients with the greatest degree of liver damage — those with advanced cirrhosis of the liver — the companies are mostly paying for the medication except where there are exceptional circumstances.” Some people have other more urgent needs, Dr. Saag says, including vasculitis, kidney disease and other widespread inflammation in the body, which is a result of an inflamed liver.
High Costs of New Hep C Drugs
Four new Direct-Acting Antiviral Agents (DAAs) have been approved by the FDA in the past 18 months. While all are being touted as highly effective, they all come with hefty price tags, placing these beneficial drugs out of reach for many patients.
Harvoni: (Approved by FDA in October 2014) Pharmaceutical company Gilead’s drug brings a cure rate of between 94 and 99%. Its 1-pill-a-day regimen makes compliance easy, but its high cost is its biggest problem — $95,500 for a 12-week regimen (though some patients may need only an 8-week treatment, doctors say. Others with more advanced cases, however, may require 24 weeks of treatment). CVS recently made a deal to be the exclusive distributor of the drug, and to reduce the price slightly.
Sovaldi: (FDA approved Sovaldi in December 2013) Another Gilead drug, sofosuvir costs about $84,000 for a 12-week course, but has to be combined with another oral medication (lidepasvir).
Olysio: (Received FDA approval in November 2013) A combination of sofosvuvir and simeprevir, Olysio costs about $66,000 per treatment and is distributed by Johnson & Johnson.
Viekira Pak: (FDA approved this combination medication in December 2014) With this latest addition to the market by AbbVie, patients must take 2 pills per day of the ombitasvir/paritaprevir/ritonavir with dasabuvir. It costs $83,319 for the 12-week course, although Express Scripts has made a deal with the manufacturer to be the exclusive distributor, and will offer a slightly discounted price.
Dr. Saag adds that he expects the market will eventually temper the cost — when more drugs become available, the price will come down in response to competition. In the meantime, some patients may have to wait for the market to adjust.
How Many Will Benefit?
Doctors now want to find those infected with HCV both recently and years ago and treat them because, for the first time, they believe a relatively easy cure is available. A simple blood test will reveal the virus. According to the American Association for the Study of Liver Diseases (AASLD), only half of those infected with the virus even know they have it.
That’s because HCV usually has no symptoms, but it silently takes over your liver, leading many patients to develop cirrhosis, liver cancer or other life-threatening liver diseases. “Untreated, 30 to 40% of these cases will progress to scarred liver disease or end up with end-stage liver disease,” says Dr. Saag. (A Hep C FAQ is available from the CDC.)
Hepatitis C develops when an individual picks up the virus through contact with the blood of an infected person. The infection leads to inflammation of the liver. Since the liver performs many functions — from processing blood to filtering toxins to producing bile to aid digestion — inflammation of the liver can have a negative impact on many parts of the body, including the stomach, gall bladder, thyroid, blood, brain and others areas as well.
 Baby boomers — those born between 1945 and 1965 — comprise the highest risk group for the disease. Here’s why: Before 1992, the blood and organ supply was not routinely checked for HCV, so if you had a transfusion or organ transplant before that date, you are at risk. If you injected intravenous drugs and shared needles with fellow users even once, you could have been exposed to the virus. Even if you snorted drugs, you could be at risk because small amounts of blood may remain on a drug straw and enter your body through microscopic tears in your nose.
Baby boomers — those born between 1945 and 1965 — comprise the highest risk group for the disease. Here’s why: Before 1992, the blood and organ supply was not routinely checked for HCV, so if you had a transfusion or organ transplant before that date, you are at risk. If you injected intravenous drugs and shared needles with fellow users even once, you could have been exposed to the virus. Even if you snorted drugs, you could be at risk because small amounts of blood may remain on a drug straw and enter your body through microscopic tears in your nose.
Other risks include having been treated with a blood-clotting product before 1987. Healthcare workers who were stuck by a needle or splashed with contaminated blood may be at risk, as is anyone who’s had unprotected sex with someone who has HCV.
While many people today are aware of those risks and avoid them, HCV is on the rise among young homosexual men who have unprotected sex and populations in non-urban areas who are using needles to inject crystal meth or heroin, notes Henry Masur, MD, Chief, Critical Care Medicine Department at the National Institutes of Health.
Until There’s a Vaccine…
Dr. Masur says insurance companies may realize that performing liver transplants and treating people with liver cancer are also very expensive propositions. “We don’t want to wait until patients have these late-stage complications,” he says. “We want to treat them early before they develop these morbidities. The only way to prevent it is to change behavior — reduce substance abuse with IV drugs, offer clean needles to drug users and make sure that men who have sex with men use condoms. Prevention is something we have to do. There is no vaccine for Hep C.”
For more information
We Now Have the Cure for Hepatitis C, but Can We Afford It? (Scientific American)
Hepatitis C: The Basics (hepmag.com)
EU Approves Drug That Is Up to 100% Effective Against Hep C (hepatitiscentral.com)
Pros and Cons of New Hepatitis C Treatments for Patients (Everyday Health)
New Drugs Offer Easier Effective Hepatitis C Treatment (Harvard Health Publications)
Additional Resources
Hepatitis C (World Health Organization)