You and I are more likely to purchase a product with labels that include “healthy,” “organic” and “all natural” according to market research. Millennials want to see those words, and even more, they want to know the source of their food.
It doesn’t come as a shock to many of us that there can be misleading information when it comes to marketing and labels on products. So how do we find the healthy products we want?
Is The FDA In Charge?
It’s name is the Food and Drug Administration (FDA). It is empowered to keep our food safe, right?
The FDA’s job is to ensure that the food and drugs we buy are safe and effective. But when it comes to food, the FDA only acts if there is an immediate harm to the population (think lettuce recalls). One of the ways we are protected from misleading marketing is actually the FDA itself.
It is actually the Federal Trade Commission (FTC) is charged with monitoring false advertising. Its “truth-in-advertising” aims to create federal laws that the advertisement agency of any product must follow.
“Federal law says that ads must be truthful, not misleading, and, when appropriate, backed by scientific evidence,” said the FTC’s website.
Meanwhile, Truth in Labeling laws are generally also regulated state by state. The National Agricultural Law Center says only 13 of the 50 states have strict laws about new-to-market labeling, limiting the use of words. However, the center notes these labels often only pertain to meat products (or new protein replacement products), and not all products on the market.
A Change in ‘Healthy’ Food Labels
You may have seen recent headlines regarding the FDA’s updates to the use of the term “healthy” when making food labels. When used (compliance is voluntary, mind you), the rules and regulations for labeling a food product as “healthy” were last updated in 1994. Those rules are woefully out of date, for example the FDA’s 1994 regulations did not even consider the amount of sugar in a product, but instead relied on “calories” to disqualify any foods that were too sugary from being labeled as “healthy.”
The FDA claims that the new proposal is geared at bringing the term “healthy” (when used on a food label) up to more recent standards and guidelines.
What Will Change?
“The proposed rule would update the definition for the implied nutrient content claim ‘healthy’ to be consistent with current nutrition science and Federal dietary guidance,” reads the FDA press release.
So, what’s new enough to warrant a change in definition?
“Specifically, to meet the proposed definition, a food product would need to contain a certain amount of food from at least one of the food groups or subgroups (e.g., fruit, vegetables, grains, dairy and protein foods) recommended by the 2020-2025 Dietary Guidelines for Americans,” said the FDA’s release.
The FDA aims to limit the amount of non-food products, additives, and preservatives that can constitute a healthy food.
Additionally, the change has a goal to keep an eye on three main targets:
- Added sugars
- Saturated fats
- Sodium
contained in “healthy” foods.
“The specific limits for added sugars, saturated fat and sodium would be based on a percentage of the Daily Value for these nutrients,” said the release from the FDA. “DVs are reference amounts of nutrients to consume or not to exceed each day.”
Size Will Matter
The newly restricted amounts will vary, depending on what type of food category the product falls into, such as its food group, type, and size.
“The proposed criteria for how much food from a particular food group is required (called food group equivalents) and the specific limits for the three nutrients vary for individual food products, mixed products (which contain more than one food group), main dishes and meals, and are based on a Reference Amount Customarily Consumed, which is the basis for determining a serving size,” said the FDA
Fresh fruits and vegetables will be permitted to be called “healthy” by definition, “because of their nutrient profile and positive contribution to an overall healthy diet,” said the FDA. However, most colorful cereals marketed to children, which given previous guidelines would have been allowed, will now be excluded, since the sugar restrictions will put them over the allowed limits.
“Natural” Means Nothing, and That’s The Law
According to the Mayo Clinic, the term “natural” represents ingredients that are “directly derived from nature and not created in a lab.” However, FDA has never legally defined the term and has no regulations on its use on food labels.
According to its website, the FDA “has considered the term ‘natural’ to mean that nothing artificial or synthetic (including all color additives regardless of source) has been included in, or has been added to, a food that would not normally be expected to be in that food.”
Instead of outlining a direct answer, despite three Citizen Petitions and even federal court litigations on the matter, thus far the FDA simply states that it “has not engaged in rulemaking to establish a formal definition for the term ‘natural.’”
‘Organic”’ Means Something… Sometimes
Another buzz word often noted in the food, drug, and cosmetics industries is “organic.” By Merriam-Webster definition, organic means that a product is “produced (or involving production) without the use of chemical fertilizers, pesticides, or other artificial agents.”
The term “organic” itself is not regulated by the FDA, at all. The USDA (US Department of Agriculture) is responsible for organic claims on food labels, supplements, and cosmetics. However, only the agricultural ingredients are regulated, not the product’s additives.
For example, processed organic foods may contain some approved non-agricultural ingredients, like the enzymes found in yogurt. You also may find an “organic” jam, but the pectin in fruit jams are not an agricultural product. Likewise, baking soda in baked goods is not organic, but the wheat grown to make it might be.
The USDA relies on the National Organic Program (NOP) to enforce the guidelines when it comes to using “organic” in product labels. NOP is the federally regulated “governing organically produced crops and livestock.”
Any product that can meet the USDA’s definition and has been reviewed by its board can use the officially approved logo.

Still No Label Requirements
This definition, however, does not stop the industry from misusing phrases such as “natural” organic,” “organic skincare,” or produced via “organic farming.” For your piece of mind, you can search for food products that include the USDA official seal, knowing it is certified as organic. Some companies cleverly make up labels that say “Organic”, but without the USDA Organic seal, it’s just made up.
Stating your product is “organic” isn’t even illegal. It is only false advertising if you use the official seal (seen above).
“If a manufacturer claims a product to be organic, but that product does not carry an official seal, the product may not meet USDA organic standards,” warns the Mayo Clinic. Looking for the official seal, as issued by the USDA, can be one of the only ways to know if a product is actually organic.
What’s It All Mean?
If you can’t trust organizations like the FDA and the USDA to regulate your food and drugs, who can you trust? While the organizations aim to clarify such terms, some efforts have only made the situation more difficult. Clearly the misuse and exaggeration of labels intended to help have only muddied the waters.
You can also always consult your personal care professionals to discuss what products to look for (and perhaps to stay away from, too).
Read Your Labels
Rather than trusting the buzzwords on the front of the package, try turning it around and reading the more detailed nutritional information and list of ingredients on the pack. While these labels are also regulated by the FDA, there are strict guidance and laws that all food manufacturers must follow.
The Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Fair Packaging and Labeling Act are the Federal laws governing food products under FDA’s jurisdiction. The FDA, however says that “it is recommended that manufacturers and importers become fully informed about the applicable laws and regulations before offering foods for distribution in the United States.”
The FDA does regulate both imported and American-manufactured products for consumption. The Nutrition Labeling and Education Act (NLEA), which amended the FD&C Act, requires “most foods to bear nutrition labeling and requires food labels that bear nutrient content claims and certain health messages to comply with specific requirements.”
This act notes that there are many changes constantly happening with requirements. It also puts the responsibility largely on the food industry itself to stay on top of these changing regulations.
“It is the responsibility for the food industry to remain current with the legal requirements for food labeling,” said the Congressional Act.
For those wishing to stay atop of updates, including all new regulations, information is published in the Federal Register website found here.
Nutritional labels are designed to inform you about what is inside a packaged food. Learn how to read them appropriately.
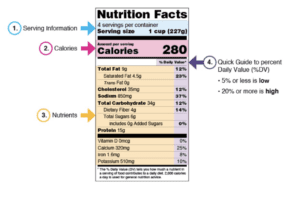
Be sure to note how many servings are included in a package. If you aren’t sure what an unpackaged food (i.e. fresh produce, handmade goods, etc.) might contain, research the product online or ask the maker.
It’s Up to You
In the end, it is really up to you as the consumer to make choices for yourself. Make sure you have a balanced diet or have spoken to your healthcare provider about diets that may help any medical conditions you may have.
Don’t believe everything you read. Flashy labels spouting phrases that seem too good to be true just might be that! Educate yourself, and advocate for yourself, to be sure you are getting the food, drugs, supplements, and self-care products that are safe, healthy, and good for you! Knowing the differences yourself is the best defense against confusing terms and labels.






