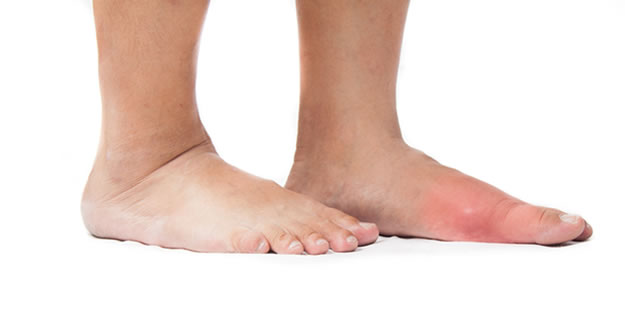
The gout drug Uloric (febuxostat) appears to have a higher rate of heart-related death when compared with another gout medication, according to a new FDA safety alert.
Following Uloric’s approval in 2009, the FDA required its manufacturer, Takeda Pharmaceuticals, to conduct a postmarket safety trial because clinical trials indicated that patients treated with Uloric had a higher rate of heart-related issues – such as heart attacks, strokes and heart-related deaths – compared to patients taking Zyloprim or Aloprim (allopurinol).
The warnings and precautions section of Uloric’s label notes the risk of cardiovascular events.
The safety trial enrolled 6,000 gout patients treated with either febuxostat or allopurinol. Preliminary results showed there was a higher risk of heart-related death and death from all types of causes in the febuxostat group.
The FDA is not taking any direct action at this time. However, it is urging doctors to consider the results before prescribing Uloric or continuing patients on the drug.