Most Recent Updates:
Click the dates to jump to more details.
- May 17, 2024: Astrazeneca is facing two lawsuits. One is a class action lawsuit from patients who experienced thrombosis after receiving the shot, and another is from a woman who was a clinical trial participant in the US. She says the company failed to support her after she suffered debilitating vaccine side effects such as nerve damage and double vision.
- July 3, 2023: Researchers are studying a possible link between vaccination and long COVID-like symptoms in a small minority of patients. These symptoms include experiences such as fatigue, irregular heart rates, and headaches that last for weeks after vaccination. Read more here.
- Feb 8, 2023: A booster shot of Moderna (the third shot, which is the same as the first two and is not the bivalent booster) was associated with spontaneously developing hives 8-13 days after the shot, that lasted six weeks or longer, according to a study in JAMA. The study suggested that 19-24 out of every 100,000 people vaccinated experienced the side effect.
- Jan 13, 2023: Prizer’s Omicron (bivalent) booster seemed to be associated with a heightened risk of stroke in patient 65 and older for three weeks after getting the shot, according to data from the Vaccine Safety Datalink, a federal safety surveillance system. The CDC and FDA released a joint statement that several other streams of data including: the Centers for Medicare and Medicaid Services database, The Vaccine Adverse Event Reporting System (VAERS), the Veteran’s Affairs (VA) database, and data from other countries did not show an association between the shot and strokes, and that they believe the vaccine is safe. Moderna’s Omicron (bivalent) booster did not show similar signals.
- July 15 2022: Of people with regular menstrual cycles 42% reported heavier periods after receiving Covid vaccines. The experience was more common among individuals with PCOS, endometriosis, fibroids or other hormonal conditions. Another 44% of those who did not have regular cycles (due to long-acting contraceptives or gender affirming treatments) reported breakthrough bleeding.
- June 14 2022: Researchers compared the adverse effects experienced by patients who took Pfizer or Moderna’s mRNA vaccine over the course of 42 weeks. The risk was very low for both, but those that took Pfizer’s were slightly more likely to suffer heart attacks or strokes. The researchers say there is a possibility that these outcomes are not linked to the vaccines, but to Covid infections, and that the results could also belie a slightly lower efficacy of Pfizer’s vaccine. They emphasize the benefits of both vaccines still outweigh the risks.
- May 6: The FDA has limited the use of the J&J vaccine after 60 cases of blood clots associated with thrombocytopenia syndrome have been discovered. 18.7 million doses of the shot have been administered, and regulators don’t yet know who is at the highest risk of clots.
- January 25: COVID vaccination did not impact the likelihood of couples conceiving in a recent study.
- January 21: The NIH is investigating the possibility that the vaccines cause long covid-like symptoms such as irregular heart rates and brain fog in very rare cases.
- January 7: Moderna and Pfizer shots are associated with a temporary increase in menstrual cycle length of just under a day, according to a new study. The researchers say future studies will look at breakthrough bleeding and heavy flows, which have been reported anecdotally.
- January 3: The FDA has authorized third shots of the Pfizer vaccine for teens 12-15 years old, and immunocompromised children between 5-11 years old. The agency also shortened the amount of time between the second and third shots from six months to five months.
- December 31: CDC data shows that serious side effects of Pfizer’s shot are extremely rare in children 5-11. While many reported arm pain, fevers and muscle pain like the adults, only 11 out of more than 40,000 reported myocarditis. Seven of those have fully recovered and four are currently improving.
- October 27: MedShadow reached out to cardiologist Jennifer Su, MD, to help you understand the risk of myocarditis in kids and teens who receive the COVID-19 vaccines. Read the article here.
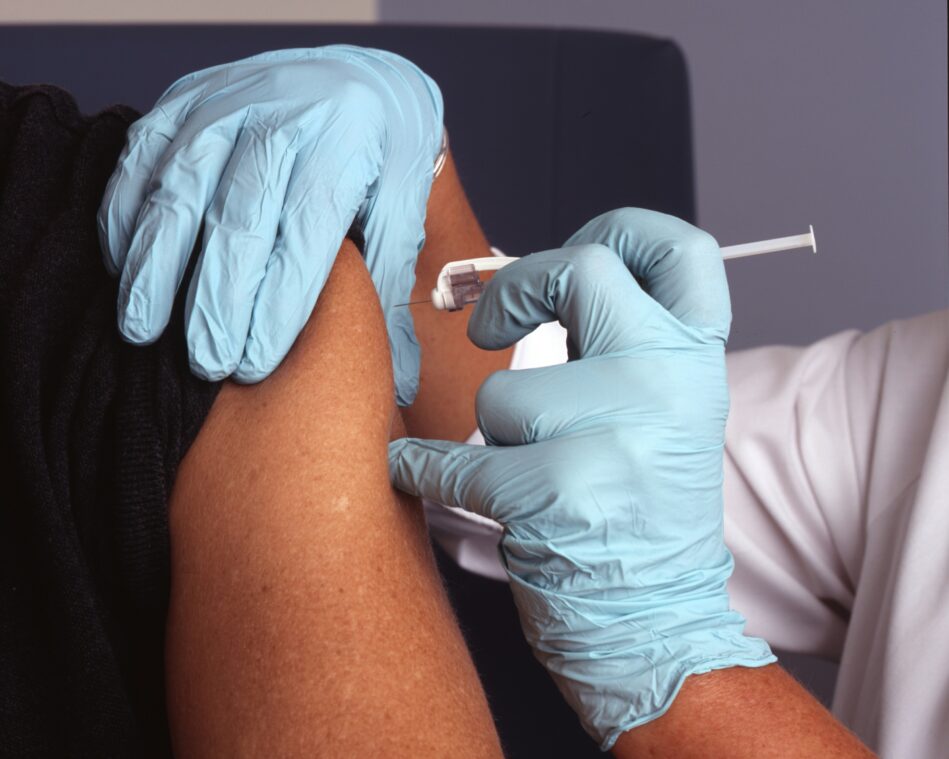
To help you sort through the news on the COVID-19 vaccine’s progress, MedShadow has created the MedShadow Vaccine Tracker, the only tracker focused on the side effects and adverse events associated with proposed COVID-19 vaccinations.
Rather than injecting patients with a weakened virus or proteins from the pathogen that our immune systems can recognize, which is what’s typically done when we get a flu vaccine, the new vaccines from Moderna and Pfizer — the two that made it to the market first in the U.S. — contain mRNA (messenger RNA), which is a genetic template that instructs our cells to build the viral proteins that our immune systems can then recognize. Its main perk is that allowing our bodies to produce the proteins (rather than growing them in a lab like traditional vaccines) slashes production time. For nearly 20 years, researchers have been interested in using mRNA in vaccines; and some were even tested in early clinical trials for rabies, influenza and Zika. However, the vaccines for COVID-19 will be the first mRNA vaccines ever authorized by the FDA.
Some risks and minor side effects, such as a sore arm where the vaccine is injected or a light skin rash, are clearly worth the benefit of being protected against a disease. Where to draw the line at what is or is not acceptable is a personal decision. That’s where the MedShadow Vaccine Tracker can help.
On the tracker, we will be publishing up-to-date Phase 3 results information about the risks of each vaccination. Phase 3 tests the vaccine for safety and efficacy in large groups of people (tens of thousands) and is the last stage before the FDA considers approval for use in the population at large. We continue following verified reports as the vaccines are offered to the general public. It is important to note that reports of events occurring after vaccination are not inherently linked to it. As countries begin to vaccinate hundreds of millions of people, some would inevitably be diagnosed with illnesses or pass away each day with or without the injections.
Jump to:
Moderna | Pfizer | CanSinBio (China) | Gameleya (Russia) | Johnson & Johnson
Astrazeneca | Novavax (UK) | Sinopharm (China) | Sinovac (China) | Murdoch (England)|Bharat Biotech
Moderna
Moderna started Phase III clinical trials for its vaccine candidate in July. In earlier trials, nearly half of patients experienced common adverse effects like injection site pain, rash, headaches, muscle soreness, nausea and fevers after the second injection. These effects generally subsided within two days. CNBC spoke to a few individuals, some participating in Moderna’s trial and some in Pfizer’s trial who said much the same thing: the side effects were intense and included a high fever, body aches, bad headaches and exhaustion, but were worth it for protection from Covid-19.
In the FDA report published in December, the most common side effects were pain at injection site (91.6% of patients), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%). Three patients experienced Bell’s Palsy, a sudden, and usually temporary, weakening or paralysis of the facial muscles.
The CDC reports that 11% of patients experienced swollen lymph nodes after the first shot. That raised to 16% after the second shot.
A few patients with facial fillers experienced swelling after receiving the vaccine. They were treated with antihistamines and steroids.
January 18: In California, officials halted the use of one particular batch of Moderna vaccines (lot 41L20A) after a small cluster (fewer than 10) of patients at one particular site experienced allergic reactions that required medical attention.
February 1: A study posted on Feb 1 showed that patients who received the vaccine after having been previously infected with COVID-19 showed greater immune response to the first shot and more intense side effects that are associated with strong immune responses like fever and muscle aches. The study included patients who received either the Moderna or Pfizer vaccine. Some scientists believe these patients may only need a single shot to provide sufficient immunity, but more research is needed.
February 12: Out of the first 7.5 million doses administered from Dec 14- Jan 18, 19 cases of anaphylaxis were reported to VAERS after the Moderna vaccine. No patients have died from anaphylaxis. Patients are now being monitored for 15-30 minutes after receiving the vaccine to watch for signs of anaphylaxis. The CDC suggests that anyone who has an immediate allergic reaction to a single dose of an mRNA vaccine (Moderna or Pfizer) should not get the second dose. If your reaction was not immediate, you may be referred to an allergy specialist. The vaccines do not contain polysorbate, but do have a related ingredient, polyethylene glycol (PEG). If you have an allergy to either of these chemicals, you should not get the vaccines.
Many patients are reporting injection site reactions that show up shortly after the injection or up to a week later. These reactions — which are characterized by swelling, redness, itching, rashes, heat and pain — are expected to last a day to a week. Physicians emphasize that while these effects can be scary, they are not dangerous and should not prevent someone from getting the second shot. So far, doctors do not report seeing these reactions after the second shot, however so few have been given so far that scientists are not sure how common it will be on round two.
March 3: Allergy researchers at Mass General Hospital created a registry for healthcare professionals to report immediate and delayed reactions to COVID-19 vaccinations. On March 3, the researchers published a letter in the New England Journal of Medicine describing a series of 12 delayed injection site reactions including swelling and rashes. The researchers wrote that one patient received antibiotics although they were not necessary. Several others were treated with steroids or anti inflammatories. The letter included some photos and said that the reactions cleared up within a median of six days. Patients were encouraged to receive their second dose. Half of them did not experience the reaction the second time. A quarter did, but to a lesser degree.
March 8: Researchers from Mass General Brigham published a Research Letter in JAMA analyzing allergic reactions in employees who received their shots there. Out of 52,805 participants, 2.1% experienced some kind of allergic reaction, including hives, itching, rash, swelling or respiratory symptoms within the first three days after vaccination. Allergies were slightly more common with the Moderna vaccine than the Pfizer vaccine (2.2% compared to 1.95%). Sixteen experienced anaphylaxis.
Moderna has announced that it will begin testing its vaccine in children and adolescents, who they believe may have stronger immune responses, leading to more intense side effects.
April 20: Researchers have received over 25,000 responses to an online survey regarding menstrual changes after receiving any COVID-19 vaccine. The survey is ongoing as researchers hope to gain a better understanding of future directions of research. An op-ed in the New York Times explains how the vaccines could potentially interact with menstrual cycles, and why we really don’t know yet.
April 22: The CDC published an analysis of data from the first few months of voluntary V-safe surveys that suggested that the Moderna and Pfizer vaccines are safe for pregnant women. Pregnant patients self-reported slightly fewer side effects like fever, headache and chills than the general population, but more said they experienced nausea and injection site pain. Rates of adverse pregnancy outcomes such as pre-term birth were similar to rates seen before the pandemic. While promising, officials noted that more studies will be required.
April 26: MedShadow asked doctors about some of the unusual symptoms we’re hearing about in the comments: heart palpitations and visual disturbances. They hadn’t had patients who reported long-term symptoms, but said that if the symptoms occurred very quickly after vaccination, they could be related to allergic reactions or presyncope.
April 27: Clinical trials of Moderna and Pfizer vaccines showed 7 cases of Bell’s Palsy. An analysis published today in JAMA suggested the experience was no more frequent after these vaccines than other vaccines. It is unclear whether vaccines raise the risk of facial paralysis at all, but the authors write, “if an association between facial paralysis and mRNA COVID-19 vaccines exists, the risk is likely very low, as with other viral vaccines.”
May 5: Researchers published a detailed analysis of skin reactions after Moderna and Pfizer vaccinations, including swelling and rashes. Most patients with first dose reactions did not have reactions after their second dose. Larger reactions on the arm spreading outward from the injection site were the most common reactions among those who received the Moderna vaccine, followed by smaller localized reactions and hives. Five patients (1.9%) experienced a zoster (shingles) flare after the first dose but none after the second dose. About 1% of patients experienced a flare of an existing skin condition after each dose.
May 20: An analysis published in JAMA suggests that hearing loss is NOT a side effect of the Pfizer or Moderna vaccines.
June 10: The CDC says that as of May 31, VAERS had received 475 reports of myocarditis or pericarditis among people 30 years of age and younger who received a COVID-19 vaccine. Upon follow-up, the CDC and FDA confirmed 226 of the reports. They emphasize that more than 18 million people between ages 12-24 have received at least one dose of COVID-19 vaccine in the United States. A CDC Fact Sheet on the effect is available here.
June 23: About 1.9% of healthcare employees who received an mRNA vaccination had a skin reaction such as itching or a rash after the first dose. Most (83%) who reported a skin reaction after the first dose, did not experience it again after the second dose, according to a study today in JAMA Dermatology. Another 2.3% experienced a skin reaction after only the second dose.
August 18: The FDA and CDC authorized third shots for patients with specific conditions that compromise their immune systems. The Biden administration has also pushed for booster shots to be made available to everyone, eight months after their second dose, in response to waning antibody levels and a rise in breakthrough infections. People who took Pfizer, Moderna or J&J’s vaccines are still highly protected against hospitalization and death. This plan is not yet authorized. The agencies are also waiting for data to provide guidelines specific to J&J vaccine recipients in the coming weeks. Around a million people have gotten third shots so far in the US and abroad. The FDA hasn’t reviewed all of the data yet, but no new safety concerns have emerged thus far. See answers to frequently asked questions regarding the booster shots here.
August 19: The FDA and CDC are investigating the possibility that Moderna’s vaccine could be up to 2.5 times more likely to be associated with myocarditis (heart inflammation), especially for males under 30 years old. If true, the effect would still be exceedingly rare and scientists say the benefits still greatly outweigh the harms of vaccination. Most patients recover on their own with minimal intervention.
October 23:Researchers found that allergies to the mRNA vaccines are not likely due to the chemical PEG. Most patients (86.7%) who experienced an immediately allergic reaction after the first shot could safely receive the second dose if pretreated with antihistamines. None of the patients required hospital admission.
October 27: MedShadow reached out to cardiologist Jennifer Su, MD, to help you understand the risk of myocarditis in kids and teens who receive the COVID-19 vaccines. Read the article here.
Dec 8: The FDA authorized Astrazeneca’s monoclonal antibody treatment as an alternative to the vaccine for people over 12 years old who are immunocompromised or have a history of severe vaccine reactions. The treatment is given in two injections and protection is thought to last 6 months up to a year. It reduced the risk of symptomatic COVID-19 by 82%.
June 14: Researchers compared the adverse effects experienced by patients who took Pfizer or Moderna’s mRNA vaccine over the course of 42 weeks. The risk was very low for both, but those that took Pfizer’s were slightly more likely to suffer heart attacks or strokes. The researchers say there is a possibility that these outcomes are not linked to the vaccines, but to Covid infections, and that the results could also belie a slightly lower efficacy of Pfizer’s vaccine. They emphasize the benefits of both vaccines still outweigh the risks.
Feb 8, 2023: A booster shot of Moderna (the third shot, which is the same as the first two and is not the bivalent booster) was associated with spontaneously developing hives 8-13 days after the shot, that lasted six weeks or longer, according to a study in JAMA. The study suggested that 19-24 out of every 100,000 people vaccinated experienced the side effect.
July 3, 2023: Researchers are studying a possible link between vaccination and long COVID-like symptoms in a small minority of patients. These symptoms include experiences such as fatigue, irregular heart rates, and headaches that last for weeks after vaccination. Read more here.
Pfizer
Pfizer began Phase III clinical trial for its vaccine candidate in July. In earlier trials, some patients experienced common adverse effects like injection site pain, rash, headaches, muscle soreness, nausea and fevers. These effects generally subsided within two days. CNBC spoke to a few individuals, some participating in Pfizer’s trial and others in Moderna’s trial who said much the same thing: the side effects were intense and included a high fever body aches, bad headaches and exhaustion in addition to the more common side effects, but were worth it for protection from Covid-19.
Some patients described the side effects as being similar to a bad hangover. A nurse who participated in the clinical trial reported feeling minimal effects after the first dose, but a fever that reached more than 104 degrees Fahrenheit after the second injection, along with chills, headache, and intense injection site pain. According to researchers, her experience of having all symptoms together was rare, though many patients had one or two of these side effects. “Clinicians will need to be prepared to discuss with patients why they should trust the vaccine and that its adverse effects could look a lot like COVID-19,” the nurse wrote on Dec 7.
The FDA report published in December said the most common reactions were injection site reactions (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%). Swollen lymph nodes occurred in 0.3% of patients. The FDA reported that four patients who received the vaccine experienced Bell’s Palsy.
January 12: A doctor in Florida died 16 days after receiving the vaccine from a rare blood disorder, acute immune thrombocytopenia. Both Pfizer and the CDC are investigating, though the company has released a statement that so far, they haven’t seen any signs in the clinical trials or data collected from early vaccinations that the death could be related to the vaccine. The blood disorder immune thrombocytopenia, has also been seen as a rare complication of COVID-19 itself, in both symptomatic and asymptomatic patients.
January 19: Twenty-three elderly patients in Norway died after receiving Pfizer’s vaccine. Officials are investigating whether or not the deaths are vaccine-related. Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, suggests that while the deaths may be coincidental given the fragile population, it’s possible that side effects of the vaccines, like fevers, may have strained the frail patients’ bodies and contributed to their deaths. Norway has vaccinated about 45,000 people so far, most of whom live in nursing homes.
February 1: A study posted on Feb 1 showed that patients who received the vaccine after having been previously infected with COVID-19 showed greater immune response to the first shot and more intense side effects that are associated with strong immune responses like fever and muscle aches. The study included patients who received either the Moderna or Pfizer vaccine. Some scientists believe these patients may only need a single shot to provide sufficient immunity, but more research is needed.
February 12: Out of the first 9.9 million doses administered from Dec 14- Jan 18, 47 cases of anaphylaxis were reported to VAERS after the Pfizer vaccine. Patients are now being monitored for 15-30 minutes after receiving the vaccine to watch for signs of anaphylaxis. The CDC suggests that anyone who has an immediate allergic reaction to a single dose of an mRNA vaccine (Moderna or Pfizer) should not get the second dose. If your reaction was not immediate, you may be referred to an allergy specialist. The vaccines do not contain polysorbate, but do have a related ingredient, polyethylene glycol (PEG). If you have an allergy to either of these chemicals, you should not get the vaccines.
March 8: Researchers from Mass General Brigham published a Research Letter in JAMA analyzing allergic reactions in employees who received their shots there. Out of 52,805 participants, 2.1% experienced some kind of allergic reaction, including hives, itching, rash, swelling or respiratory symptoms within the first three days after vaccination. Allergies were slightly more common with the Moderna vaccine than the Pfizer vaccine (2.2% compared to 1.95%). Sixteen experienced anaphylaxis.
March 30: Researchers publish a case study of a single patient in which they suspect the Pfizer vaccine may have caused a rheumatoid arthritis flare. The flare was treated, and the patient is in remission. The researchers and RA advocacy groups still advise patients to get the vaccine as the benefits outweigh the risks of a flare up.
April 20: Researchers have received over 25,000 responses to an online survey regarding menstrual changes after receiving any COVID-19 vaccine. The survey is ongoing as researchers hope to gain a better understanding of future directions of research. An op-ed in the New York Times explains how the vaccines could potentially interact with menstrual cycles, and why we really don’t know yet.
April 22: The CDC published an analysis of data from the first few months of voluntary V-safe surveys that suggested that the Moderna and Pfizer vaccines are safe for pregnant women. Pregnant patients self-reported slightly fewer side effects like fever, headache and chills than the general population, but more said they experienced nausea and injection site pain. Rates of adverse pregnancy outcomes such as pre-term birth were similar to rates seen before the pandemic. While promising, officials noted that more studies will be required.
April 26: MedShadow asked doctors about some of the unusual symptoms we’re hearing about in the comments: heart palpitations and visual disturbances. They hadn’t had patients who reported long-term symptoms, but said that if the symptoms occurred very quickly after vaccination, they could be related to allergic reactions or presyncope.
April 27: Clinical trials of Moderna and Pfizer vaccines showed 7 cases of Bell’s Palsy. An analysis published today in JAMA suggested the experience was no more frequent after these vaccines than other vaccines. It is unclear whether vaccines raise the risk of facial paralysis at all, but the authors write, “if an association between facial paralysis and mRNA COVID-19 vaccines exists, the risk is likely very low, as with other viral vaccines.”
May 5: Researchers published a detailed analysis of skin reactions after Moderna and Pfizer vaccinations, including swelling and rashes. Nearly a quarter of patients had smaller local reactions after each of the two shots. Hives were the most common skin reaction (26% after the first shot, 18% after the second,) among patients who received the Pfizer vaccine. Several also experienced flares of existing skin conditions.
May 10: Pfizer’s vaccine received Emergency Use Authorization for 12 to 15-year-olds. The company reported that common adverse effects like chills and fever were more common in this age group than adults. Adverse reactions in adolescents 12 through 15 years of age included pain at the injection site (90.5%), fatigue (77.5%), headache (75.5%), chills (49.2%), muscle pain (42.2%), fever (24.3%), joint pain (20.2%), injection site swelling (9.2%), injection site redness (8.6%), lymphadenopathy (0.8%), and nausea (0.4%). These effects generally subsided within 1-2 days. While data on syncope (fainting) was not included, the announcement mentioned that fainting after vaccination is more common in teens than adults, and that safety measures should be in place to reduce this risk.
May 20: An analysis published in JAMA suggests that hearing loss is NOT a side effect of the Pfizer or Moderna vaccines.
May 23: The CDC is investigating reports of myocarditis in a small number of vaccinated teens. Myocarditis, or inflammation of the heart, has multiple causes but a viral infection is the most common trigger. In 2019, one million people were diagnosed with myocarditis, most of them under 40 years old. It’s not yet clear if these events were related to the vaccine.
June 9: A study in Nature Medicine suggests that there may be a very slightly elevated risk of blood disorders after receipt of the AstraZeneca vaccine, but NOT Pfizer’s. The study was based on an analysis of medical records in Scotland. Researchers say the vaccine’s benefits still outweigh the risks and that their results should not alter policy. The rates are similar to those seen with other common vaccines.
June 10: The CDC says that as of May 31, VAERS had received 475 reports of myocarditis or pericarditis among people 30 years of age and younger who received a COVID-19 vaccine. Upon follow-up, the CDC and FDA confirmed 226 of the reports. They emphasize that more than 18 million people between ages 12-24 have received at least one dose of COVID-19 vaccine in the United States. A CDC Fact Sheet on the effect is available here.
June 23: About 1.9% of healthcare employees who received an mRNA vaccination had a skin reaction such as itching or a rash after the first dose. Most (83%) who reported a skin reaction after the first dose, did not experience it again after the second dose, according to a study today in JAMA Dermatology. Another 2.3% experienced a skin reaction after only the second dose.
August 18: The FDA and CDC authorized third shots for patients with specific conditions that compromise their immune systems. The Biden administration has also pushed for booster shots to be made available to everyone, eight months after their second dose, in response to waning antibody levels and a rise in breakthrough infections. People who took Pfizer, Moderna or J&J’s vaccines are still highly protected against hospitalization and death. This plan is not yet authorized. The agencies are also waiting for data to provide guidelines specific to J&J vaccine recipients in the coming weeks. Around a million people have gotten third shots so far in the US and abroad. The FDA hasn’t reviewed all of the data yet, but no new safety concerns have emerged thus far. See answers to frequently asked questions regarding the booster shots here.
August 22: Researchers published two case studies of patients experiencing neurological effects such as losing balance and non-epileptic convulsions after the Pfizer and AstraZeneca vaccines. They emphasize that functional neurological disorders are treatable.
September 2: Researchers in Israel reviewed medical records of patients who had experienced Guillan-Barre Syndrome (GBS) prior to receiving Pfizer’s COVID-19 shot. Of the 579 patients, five were transferred to the hospital for neurological symptoms such as tremors or prickling sensations. Only one patient required medical care for relapse of GBS.
September 28: V-Safe data from 306 people who received their third shots showed that nearly identical numbers of patients reported side effects to the second and third shots. 79.4% and 74.1% reported local or systemic reactions, respectively, after the third dose compared to 77.6% and 76.5% who reported local or systemic reactions after the second dose, respectively.
October 23: Researchers found that allergies to the mRNA vaccines are not likely due to the chemical PEG. Most patients (86.7%) who experienced an immediately allergic reaction after the first shot could safely receive the second dose if pretreated with antihistamines. None of the patients required hospital admission.
October 27: MedShadow reached out to cardiologist Jennifer Su, MD, to help you understand the risk of myocarditis in kids and teens who receive the COVID-19 vaccines. Read the article here.
Dec 8: The FDA authorized Astrazeneca’s monoclonal antibody treatment as an alternative to the vaccine for people over 12 years old who are immunocompromised or have a history of severe vaccine reactions. The treatment is given in two injections and protection is thought to last 6 months up to a year. It reduced the risk of symptomatic COVID-19 by 82%.
Dec 31: CDC data shows that serious side effects of Pfizer’s shot are extremely rare in children 5-11. While many reported arm pain, fevers and muscle pain like the adults, only 11 out of more than 40,000 reported myocarditis. Seven of those have fully recovered and four are currently improving.
June 14: Researchers compared the adverse effects experienced by patients who took Pfizer or Moderna’s mRNA vaccine over the course of 42 weeks. The risk was very low for both, but those that took Pfizer’s were slightly more likely to suffer heart attacks or strokes. The researchers say there is a possibility that these outcomes are not linked to the vaccines, but to Covid infections, and that the results could also belie a slightly lower efficacy of Pfizer’s vaccine. They emphasize the benefits of both vaccines still outweigh the risks.
January 13, 2023: Prizer’s Omicron (bivalent) booster seemed to be associated with a heightened risk of stroke in patient 65 and older for three weeks after getting the shot, according to data from the Vaccine Safety Datalink, a federal safety surveillance system. The CDC and FDA released a joint statement that several other streams of data including: the Centers for Medicare and Medicaid Services database, The Vaccine Adverse Event Reporting System (VAERS), the Veteran’s Affairs (VA) database, and data from other countries did not show an association between the shot and strokes, and that they believe the vaccine is safe. Moderna’s Omicron (bivalent) booster did not show similar signals.
July 3, 2023: Researchers are studying a possible link between vaccination and long COVID-like symptoms in a small minority of patients. These symptoms include experiences such as fatigue, irregular heart rates, and headaches that last for weeks after vaccination. Read more here.
CanSinBio (China)
China granted the CanSinBio vaccine emergency approval prior to beginning a Phase III trial in August. In the Phase II trial, nearly three-quarters of patients reported at least one common mild adverse events including injection site pain, rash, headaches, muscle soreness, and fevers. Five people also reported vomiting.
Gamaleya Research Institute (Russia)
Gamaleya Research Institute launched Phase III trials in August. But before they got started, President Putin announced that the vaccine was approved early, however, the Phase III trials are expected to continue. In the earlier trials, almost all of the patients experienced low-grade fevers. A small number of patients reported heart palpitations. Otherwise, reported side effects were similar to other vaccines and included injection site pain, rash, headaches, and muscle soreness.
Feb 2: The group reported efficacy data along with some data on side effects in The Lancet. The most common adverse events were flu-like illness (15.2% of those vaccinated) and local reactions (5.4% of those vaccinated).
The report also states that there were six grade 3 adverse events which were not associated with the vaccination: acute sinusitis, an exacerbation of urolithiasis along with renal colic and deep vein thrombosis (both associated with pre-existing comorbidities) and extremity abscess (due to physical injury and subsequent infection of the wound surface of the soft tissues of the finger).
Johnson & Johnson
Johnson & Johnson started a Phase III trial for its vaccine candidate in September 2020, but paused it on October 12 due to an unexplained illness and remains on hold. The company announced it would restart the trial on October 26. Pauses are common during clinical trials to evaluate specific adverse events, but the company has not shared details about the illness experienced by one of the trial volunteers. In earlier trials, about 70% of patients experienced at least one mild adverse effect similar to those seen with other vaccines, including injection site pain, rash, headaches, muscle soreness, and fevers.
On Feb 24, Johnson & Johnson shared data from its Phase III trials. The data suggested that while the vaccine is slightly less protective overall (but more protective against the 501Y.V2 variant that emerged from South Africa,) than the Moderna and Pfizer vaccines, it produced fewer side effects.
The report says the most common reactions were injection site reactions (50.2%), fatigue (38.2%), headache (38.9%), muscle pain (33.2%), nausea (14.2%) and fever (9.0%). Reactions were more common in patients under 60 than over 60.
Injection site pain lasted a median of 2 days, but up to 7 days in 2.3% of patients.
April 8: A vaccination site in Colorado paused operations after 11 patients became nauseous and dizzy minutes after receiving the Johnson & Johnson vaccine. Two patients were transmitted to the hospital, but the rest were deemed healthy enough to return home. Officials said that those vaccinated at the same site should not be concerned.
April 13: The U.S. is calling for a pause on distributing the Johnson & Johnson vaccine while the FDA and CDC investigate blood clots that occurred in six patients (out of 7 million who received the vaccine so far.) One patient died and another was hospitalized. All the clots occurred in women under 50 within two weeks of receiving the vaccine. The issue is similar to the one seen with the AstraZeneca vaccine in Europe.
April 20: Researchers have received over 25,000 responses to an online survey regarding menstrual changes after receiving any COVID-19 vaccine. The survey is ongoing as researchers hope to gain a better understanding of future directions of research.
April 23: The U.S. is resuming use of the vaccine after finding only 15 total cases of blood clots out of the 7 million vaccinated. Patients will be warned about the risk at vaccination sites. Symptoms including headache, leg pain and abdominal pain occur 6-14 days after vaccination. If you have these symptoms you should seek emergency medical treatment. It is treatable with certain blood thinners.
April 26: MedShadow asked doctors about some of the unusual symptoms we’re hearing about in the comments: heart palpitations and visual disturbances. They hadn’t had patients who reported long-term symptoms, but said that if the symptoms occurred very quickly after vaccination, they could be related to allergic reactions or presyncope.
April 27: Doctors suggested that patients with a history of immune-mediated thrombocytopenia, such as heparin-induced thrombocytopenia, should not get the Johnson and Johnson vaccine (mRNA vaccines like Pfizer and Moderna are ok). If you’re at an increased risk for other types of blood clots, (because you have obesity or you smoke, for example) you do not have an elevated risk of blood clots with the J&J vaccine.
April 30: Researchers published detailed data about 12 patients who experienced blood clots in JAMA.
May 13: The CDC has identified more cases of the rare clotting disorder associated with the J&J vaccine. The total is 28 cases. Three patients have died and four are still hospitalized. It’s more common in women than in men, and the benefits still outweigh the risks of vaccination, officials say. Nine million doses of the vaccine have been delivered in the U.S.
May 26: A 37-year old woman in Belgium died from a blood clot thought to be related to the Johnson and Johnson vaccine she received. The country has stopped using the vaccine in patients under the age of 41.
July 12: The FDA is attaching a warning to the Johnson & Johnson vaccine about a slightly elevated risk of developing Guillain-Barre syndrome, which can cause paralysis, within three weeks of receiving the vaccine. About 100 cases have been identified. Most patients recovered. One 57-year-old man died. The benefits still out weight the risks of vaccination, it says.
August 22: The FDA and CDC authorized third shots for patients with specific conditions that compromise their immune systems. The Biden administration has also pushed for booster shots to be made available to everyone, eight months after their second dose, in response to waning antibody levels and a rise in breakthrough infections. People who took Pfizer, Moderna or J&J’s vaccines are still highly protected against hospitalization and death. This plan is not yet authorized. The agencies are also waiting for data to provide guidelines specific to J&J vaccine recipients in the coming weeks. Around a million people have gotten third shots so far in the US and abroad. The FDA hasn’t reviewed all of the data yet, but no new safety concerns have emerged thus far. See answers to frequently asked questions regarding the booster shots here.
Dec 8: The FDA authorized Astrazeneca’s monoclonal antibody treatment as an alternative to the vaccine for people over 12 years old who are immunocompromised or have a history of severe vaccine reactions. The treatment is given in two injections and protection is thought to last 6 months up to a year. It reduced the risk of symptomatic COVID-19 by 82%.
Dec 16: The CDC’s vaccine advisors are meeting to review data that suggests a heightened risk of clotting disorders in patients who received the J&J vaccine. Women aged 30-49 appear to be the most affected, though the condition likely impacts 1 out of 100,000 vaccine recipients. Nine have died.
May 6: The FDA has limited the use of the J&J vaccine after 60 cases of blood clots associated with thrombocytopenia syndrome have been discovered. 18.7 million doses of the shot have been administered, and regulators don’t yet know who is at the highest risk of clots.
Astrazeneca
The vaccine has been approved for use in the U.K., Argentina, India, E.U. and Mexico, but in May 2024, the company stopped manufacturing the vaccine, citing low demand.
May 17, 2024: Astrazeneca is facing two lawsuits. One is a class action lawsuit from patients who experienced thrombosis after receiving the shot, and another is from a woman who was a clinical trial participant in the US. She says the company failed to support her after she suffered debilitating vaccine side effects such as nerve damage and double vision.
March 11: Denmark has paused the use of the AstraZeneca vaccine while it investigates whether it is responsible for blood clots — one fatal — in some patients.
“It is important for us that the citizens are confident in the offer we give them and trust that we can vouch for the quality of the vaccines we have in our program. Therefore, we react promptly until we have investigated whether there is a connection between the vaccine and the possible side effects ,” officials wrote in a press release.
March 15: Several other countries (Iceland, Norway, Denmark, Ireland, the Netherlands, Italy, France and Germany) have also paused use of the vaccine while unusual clotting events are investigated. Meanwhile, Thailand, which paused its use on Friday, March 12 restarted it on Sunday, March 14. On Sunday, AstraZeneca released a statement that its own review revealed “no increased risk” of clotting issues with the vaccine and that “So far across the EU and UK, there have been 15 events of DVT [deep vein thrombosis] and 22 events of pulmonary embolism reported among those given the vaccine, based on the number of cases the Company has received as of 8 March. This is much lower than would be expected to occur naturally in a general population of this size and is similar across other licensed COVID-19 vaccines.”
March 18: The European Medicines Agency (EMA) issued a statement that the benefits of the AstraZeneca vaccine outweigh the risks. The vaccine is not associated with an overall risk of blood clots, though there may be a very small increased risk of certain types of clots. The statement reads, “These are rare cases – around 20 million people in the UK and EEA had received the vaccine as of March 16 and EMA had reviewed only 7 cases of blood clots in multiple blood vessels (disseminated intravascular coagulation, DIC) and 18 cases of CVST [clots in the vessels that drain blood from the brain.] A causal link with the vaccine is not proven, but is possible and deserves further analysis.” These cases were more common in women under the age of 55. Germany, France, Italy, Latvia, Bulgaria and Slovenia have all stated that they’d restart or continue to offer the AstraZeneca vaccine in light of the news.
April 7: The EMA still says the benefits of the vaccines outweigh the risks. However, the group has suggested that blood clots be listed as a possible, albeit rare, side effect of the injection and that both patients and doctors be aware of the signs which include: shortness of breath, chest pain, leg swelling, persistent abdominal pain, neurological symptoms like blurry vision and blood spots under the skin beyond the site of injection. If you have these symptoms, seek medical assistance.
April 9: Out of 34 million recipients of the vaccine, 222 have reported blood clots. They appear to be more likely in younger patients, but it’s otherwise impossible to predict who will experience them. Some experts say they can be treated with intravenous immune globulin, which is currently used to treat immune disorders along with some types of blood thinners.
June 9: A study in Nature Medicine suggests that there may be a very slightly elevated risk of blood disorders after receipt of the AstraZeneca vaccine, but not Pfizer’s. The study was based on an analysis of medical records in Scotland. Researchers say the vaccine’s benefits still outweigh the risks and that their results should not alter policy. The rates are similar to those seen with other common vaccines.
August 22: Researchers published two case studies of patients experiencing neurological effects such as losing balance and non-epileptic convulsions after the Pfizer and AstraZeneca vaccines. They emphasize that functional neurological disorders are treatable.
Dec 8: The FDA authorized Astrazeneca’s monoclonal antibody treatment as an alternative to the vaccine for people over 12 years old who are immunocompromised or have a history of severe vaccine reactions. The treatment is given in two injections and protection is thought to last 6 months up to a year. It reduced the risk of symptomatic COVID-19 by 82%.
Novavax (United Kingdom)
Novavax began Phase III clinical trials with its vaccine candidate in the United Kingdom in September and is had planned to start a trial in the United States in October. The trial was delayed due to manufacturing problems. The U.S. trial started on December 28. During Phase I/II trials, patients reported adverse events similar to those of other vaccine candidates, including injection site pain, rash, headaches, muscle pain, fever, nausea and vomiting.
June 14: Novavax announced promising efficacy data in a press release. The release suggested that the vaccine has similar side effects to others already approved, but that they may be slightly milder. Complete data is not yet available.
Sinopharm (China)
In July, Sinopharm launched Phase III trials of its two vaccine candidates in the United Arab Emirates, Peru, Morocco, and Argentina, which are now approved for limited use in healthcare workers in China and the UAE. In earlier trials of one of the vaccines, made in collaboration with Wuhan Institute of Biological Products, about 15% of the patients reported adverse events similar to those of other vaccine candidates, including injection site pain, rash, headaches, muscle pain, fever, nausea and vomiting. There is little data available about the second vaccine, made in collaboration with the Beijing Institute of Biological Products.
Sinovac (China)
Sinovac opened Phase III trials of its vaccine candidate, CoronaVac, in July. It is already being used with emergency approval for high risk individuals in China. The earlier trials showed that about a third of patients experienced adverse effects similar to those in other vaccine candidates like injection site pain, fever, and fatigue, but the study included few details about more specific symptoms like nausea, headaches, and vomiting. On November 10, the trial was halted in Brazil due to a participant’s death. However, researchers say the death was not vaccine-related and the trial should continue. It has not been halted in other countries.
Murdoch Children’s Research Institute (England)
The Murdoch Children’s Research Institute is running a Phase III trial repurposing Bacillus Calmette-Guerin — a vaccine developed nearly a century ago to prevent tuberculosis infections — to prevent Covid-19. Since the vaccine has been around such a long time, there are many reports on its potential adverse effects and the vaccine itself has been altered to minimize many of them. Injection site reactions are common, and lymphadenitis, swelling of the lymph nodes, is common.
Bharat Biotech
Bharat Biotech announced it would begin Phase III trials in India on October 23. In early trials demonstrated side effect profiles similar to those of the other vaccines, including pain at the injection site, fatigue, headache, and fever. The country announced emergency approval, though no Phase III data was included in the announcement.