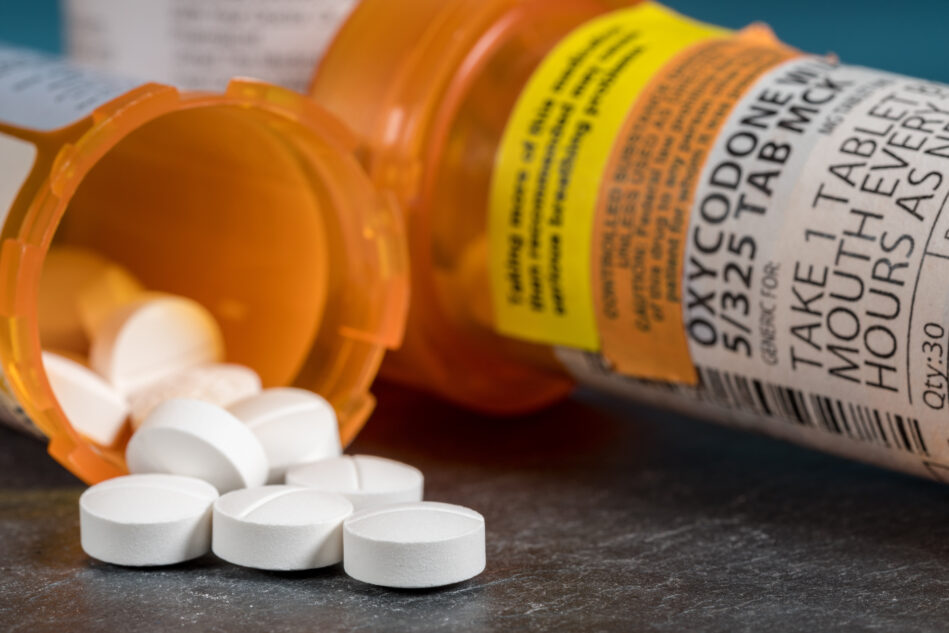
The FDA is going after 9 companies operating 53 websites for allegedly hawking online unapproved and misbranded versions of opioid medications, such as oxycodone and tramadol.
The FDA has issued warning letters — which order the companies to stop selling the products immediately — to the 9 firms. The agency noted that patients who purchase these substances may be “putting their health at risk because the products, while being marketed as authentic, may be counterfeit, contaminated, expired, or otherwise unsafe.”
The illegal online sale of epilepsy prescription opioids is particularly concerning considering that oxycodone and tramadol have boxed warnings indicating they have a significant risk of serious and potentially life-threatening adverse events. These risks include addiction, abuse and breathing problems.
“The new warning letters are part of a comprehensive campaign to target illegal sales of unapproved opioids,” FDA Commissioner Scott Gottlieb said in a statement. “We’ll be following these actions with additional steps in coming months to crack down on the flow of illegal, unapproved opioids sold online and shipped through the mail.”