Maybe you’re dealing with aches and pains, a runny nose, or an upset stomach. Do you reach for an over-the-counter medication or supplement to help combat your symptoms? And what should you look at if you’re trying to decide?
OTC drugs (Over-the-counter) are medications that are available without a prescription. They include a wide range of products, such as nonsteroidal anti-inflammatory drugs (NSAID) to treat inflammation and fever, dextromethorphan for coughs, and proton pump inhibitors (PPIs) for stomach acid. OTC drugs are regulated by the FDA (Food and Drug Administration). The FDA requires that the information on all OTC labels be listed in the same order, in an easy-to-read format.
Supplements are such things as vitamins, minerals and herbs, and can be used to help your body get enough of certain important substances it needs. Or consumers may use them in hopes of treating or warding off illnesses. However, supplements cannot be marketed for the purpose of treating, diagnosing, preventing or curing a disease, according to the FDA. Common supplements include echinacea, which is used to fight colds; St. John’s wort, which is taken to combat depression; or multivitamins.
OTC Drugs
Dr Tochi Iroku-Malize, chair of family medicine for Northwell Health, https://www.northwell.edu/about/leadership/tochi-iroku-malize-md
based in New York City, recommends looking at the inactive ingredients in an OTC, as well as the active ingredients because both can cause allergic reactions.
You should also check to be sure the OTC you take won’t interact with other medications or supplements you take, she says.
Iroku-Malize recommends that you find an OTC that addresses your particular symptoms, rather than one that treats a wide range of symptoms.
Many OTC drugs have “another side effect that does other things to the body. Just because it’s an OTC doesn’t mean it doesn’t cause problems,” she says.
OTCs are required to have a Drug Facts label, and must include the following information.
- Active ingredient – the therapeutic substance in the product and the amount of active ingredient per unit
- Uses – the symptoms the product treats or prevents
- Warnings – when you shouldn’t use the product; when you should consult with a physician or pharmacist; possible interactions or side effects; what to do if you’re pregnant or breast-feeding; keep product away from children.
- Inactive ingredients – other substances in the product, such as coloring or flavorings
- Purpose – what the product is used for
- Directions – how much of the product to take, how to take it, how often to take it and for how long to take it.
- Other information – such as how to store the product
The label of an OTC also will include:
- The product’s expiration date
- The lot or batch code of the product
- The name and address of the manufacturer, packer or distributor
- The amount of product in the package
- What you should do if an overdose occurs.
Supplements
Unlike OTC medicines, supplements do not have to be proven safe or effective. Supplements are assumed safe by the FDA and unless there are reports of harm – or if the supplement makes health claims like, “cures cancer!” – then they are free to sell you anything. (Supplements cannot claim to treat, prevent, diagnose or cure a disease or condition.)
Don’t be fooled by phrases like “Clinical studies show…” They don’t prove anything. Clinical trials can be rigorous or at the level of high school intro biology. And stay far away from unrealistic claims like, “Lose 15 pounds in a week.” The most outrageous claims are often found on weight loss, sexual and bodybuilding products.
Of course, there are a few exceptions. Some supplements are allowed by the FDA to make health claims because good quality research has been conducted that the FDA has reviewed and accepted, an example is “calcium may reduce the risk of osteoporosis.” The FDA recommends that supplements carry a Supplement Facts label, similar to a Drug Facts label, but the label recommendations are not binding.
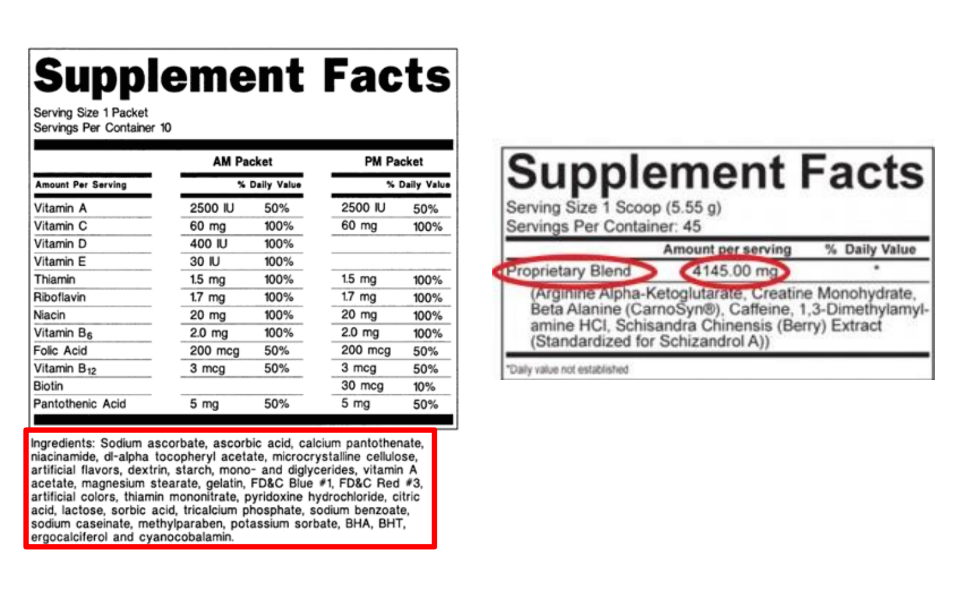
The FDA recommends supplement labels include:
- Name – the name of the product, as well as the term “dietary supplement”
- Net quantity – amount of the supplement in the package
- Nutrition labeling – names and quantities of dietary ingredients in the product; the serving size and servings per container; the part of the plant from which the ingredient is derived.
- Ingredient labeling – the compounds used in the manufacture of the product
- Name and address of the manufacturer, packer or distributor
The expiration date and product batch/lot numbers are not required for supplement labels, but having them shows accountability on the part of the company. Think carefully before buying supplements without that information.
Verified and Certified by a Third party
Pieter Cohen, a physician specializing in internal medicine and primary at Cambridge Health Alliance and an associate professor of medicine at Harvard Medical School, recommends looking for supplements that carry a third-party certification, such as from NSF International or USP.
An NSF certification verifies that the product contains what the label says it does and that it doesn’t contain unsafe levels of contaminants, such as pesticides. NSF’s Certified for Sport seal shows that the product does not contain banned substances.
USP’s certification verifies that the ingredients on the label are in the product at the listed amount and potency and doesn’t contain contaminants.
Do Your Homework
Chris D’Adamo, assistant professor of family and community medicine at the University of Maryland School of Medicine, cautions that different varieties of a particular herb or different extracts that might be used in supplements. He recommends that you choose a supplement that contains whatever herb and amount of an herb that has been used in clinical studies. D’Adamo encourages everyone to do their own research by looking on PubMed.
He also tries to avoid supplements that are proprietary blends. “You don’t know how much of each ingredient is in the product.”
D’Adamo suggests using the following websites to check on supplements you are interested in taking:
For Clarity of package ingredients, many supplements are registered with the Dietary Supplement Label Database.
For Efficacy, or if it works, check with Examine.com. Dr. D’Adamo notes that Examine.com is led by a team of scientists and is incredibly comprehensive.
For Purity, turn to ConsumerLab or Labdoor. Both companies are independent of pharmaceutical money and conduct high-quality evaluation. Neither company covers every product, but pay particular attention to those products either company gives a poor rating to or identified as problematic.
“Be sure it’s something that would be helpful to you, not harmful,” D’Adamo says.






