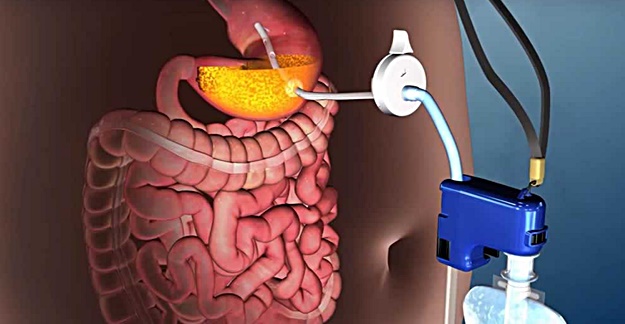
AspireAssist is essentially a pump that connects to a tube implanted in the stomach to suck food out. But is this a good way to achieve weight loss?
Overweight Americans’ battle of the bulge has lasted decades, and every time a new diet, drug or device comes out claiming it can help you lose weight, immense media attention always follows. But the FDA’s June 13 announcement that it had approved the AspireAssist device created a tsunami of coverage.
But why? Perhaps it’s because of how AspireAssist works. It’s essentially a pump that attaches to a tube that has been surgically implanted in a person’s stomach and sucks undigested food out of it after a meal and straight in your toilet bowl. Think of it as a reverse feeding tube.
Sounds gross, right? That’s because it is. But more importantly, AspireAssist may not really get to the crux of the problem for overweight individuals: Losing weight requires changes in diet (such as eating less) and lifestyle, especially regular exercise. And having a pump suck out up to 30% of the calories –- a form of mechanized bulimia — in the meal you just ate may not encourage individuals to change their eating habits at all.
by AspireBariatrics.
The device is designed to help people aged 22 and older who are obese (a body mass index of 35 to 55) and who have failed to achieve or maintain weight loss using nonsurgical therapies. However, AspireAssist is not to be used in people with eating disorders or those who are moderately overweight that want to get some short-term weight loss.
“Patients with AspireAssist therapy have the opportunity not only to lose a significant amount of weight in a safe and controlled manner, but also to develop a healthier lifestyle through more mindful eating habits,” Louis Aronne, MD, a professor of metabolic research at Weill Cornell Medicine who was one of the lead investigators of the study that led to the approval of AspireAssist, said in a statement.
Is AspireAssist a Game-Changer?
In that study, which lasted a year, 171 obese individuals were enrolled: 111 used AspireAssist along with lifestyle therapy, while the other 61 received lifestyle therapy alone. Those treated with the device and counseling lost an average of 12.1% of their body weight compared to 3.6% in the group that received counseling alone.
Sounds like a home run for obese individuals, right? Not exactly. Many doctors are shocked that AspireAssist won FDA approval.
‘This is mechanized bulimia. It’s a device that makes bulimia okay.’ — Joseph Gutman, MD
“This is the first time that I look at a device that was approved by the FDA and I am absolutely, utterly, and totally appalled that it was approved,” says Joseph Gutman, MD, a Florida endocrinologist who has treated patients with obesity for decades. He told The Verge, “Instead of throwing up through the throat, you throw up through the tube. This is mechanized bulimia. It’s a device that makes bulimia okay.”
And in 2013, Keith Ayoob, EdD, a professor of nutrition at Albert Einstein College of Medicine, expressed concern that the device was more like a “bulimia machine” since it works similarly to the purging seen in people with eating disorders.
In addition to Gutman’s and Ayoob’s concerns about the device replicating eating disorders, there are quite a number of side effects associated with AspireAssist: Occasional indigestion, nausea, vomiting, constipation and diarrhea, as well as “leakage, bleeding and/or infection around the site where the tube is placed and device migration into the stomach wall,” according to the FDA.
Only time will tell if AspireAssist catches on and becomes a commercial success. But here’s hoping that both doctors and patients will see the device as a cheat and battle obesity by treating the root of the problem to make long-lasting changes without the risk of side effects