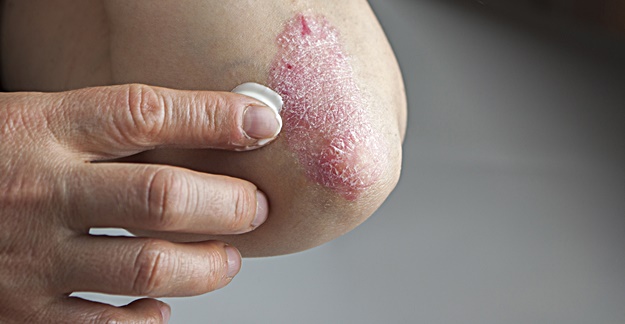
A new medication approved by the FDA in March to treat psoriasis has demonstrated sustained efficacy, even after the drug is taken for more than a year.
Taltz (ixekizumab), a biologic medication, was also found to be more effective than another popular biologic already on the market called Enbrel (etarnecept). Both medications are given by injection.
Taltz works by targeting a pathway in the immune system, called IL-17, known to promote psoriasis.
The latest findings, published in the New England Journal of Medicine, are based on results from 3 clinical trials that enrolled almost 4,000 people. In the first trial, Taltz was compared against a placebo. However, in the other two, it was compared against a placebo and Enbrel for the first 12 weeks, and then just placebo after 12 weeks.
After the first 12 weeks, the Taltz injection was given either once a month or every 12 weeks. After 60 weeks, nearly 75% of the patients given the biologic once a month had only a minimal amount of psoriasis, compared to just 7% of patients taking the placebo.
In addition, during the 12-week intial period of the studies, Taltz was more effective than Enbrel by a wide margin.
“Based on these findings, we expect that 80% of patients will have an extremely high response rate to ixekizumab, and about 40% will be completely cleared of psoriasis,” lead author Kenneth Gordon, MD, a professor of dermatology at Northwestern University Feinberg School of Medicine, said in a statement.
Like most medications, side effects were seen with some patients taking Taltz. These adverse events included slightly higher rates of low white blood cell count (neutropenia), yeast infection and inflammatory bowel disease compared to placebo. The safety of the biologic past 60 weeks of use is also not known.
Also, the research was funded by Eli Lilly, Taltz’s manufactuer. In addition, Gordon is a paid consultant for the drugmaker.