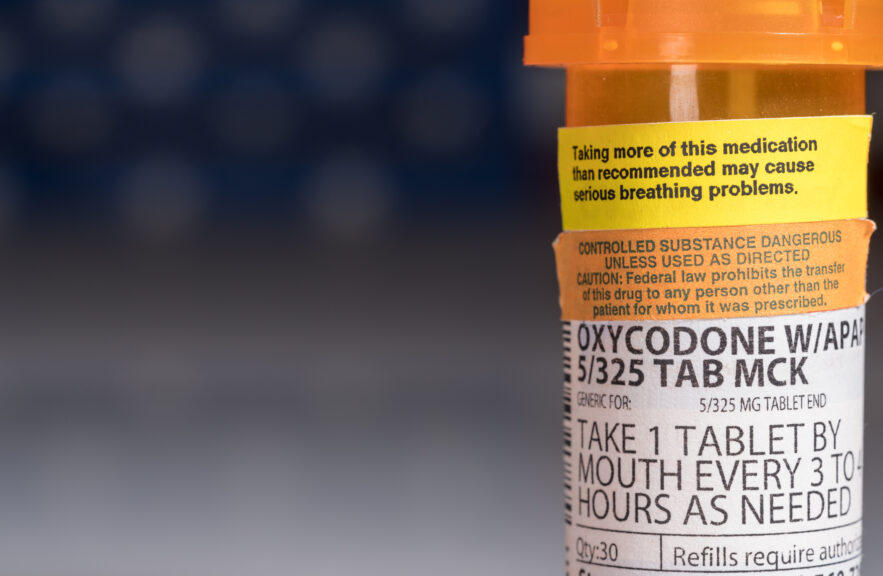
Quick Hits: “Black box” warnings – the strongest the FDA can mandate – will be added to the labeling of prescription opioid drugs and benzodiazepines amid growing concern about side effects when the medications are taken together. An FDA review found cases where people taking both drug classes experienced serious side effects, including slowed or difficulty breathing, and even death. Both opioids, which are used to treat pain, and benzodiazepines, which are tranquilizers, depress the body’s central nervous system. The agency is also advising health care professionals to limit prescribing of opioids and benzodiazepines together only to patients for whom no other alternatives are available. Posted August 31, 2016. Via FDA.
Quick Hits: The DEA is set to ban kratom, a plant-derived drug currently sold as a dietary supplement, over concerns it is addicting and has no medicinal value. The DEA wants to list kratom as a schedule I drug under the Controlled Substances Act, putting it in the same class as heroin and LSD. Kratom is known for its sedative properties, and has even been used by some as a pain reliever. There have also been anecdotal reports of people using kratom to come off dependence on prescription opioids or heroin. However, in July, the CDC noted that kratom can be abused, and between 2010 and 2015, poison control centers received more than 660 calls related to kratom. Posted August 31, 2016. Via Federal Register.
Quick Hits: The FDA has approved a biosimilar version of the autoimmune disease treatment Enbrel (etanercept). The approval is only the second time the agency has approved a biosimilar – in essence, a generic version – of a biologic based drug, which can costs tens of thousands of dollars per year. Like Enbrel, Erelzi (etanercept-szzs) is indicated to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and plaque psoriasis. Posted August 30, 2016. Via FDA.